- Trusted Wholesaler in Global Healthcare
- [email protected]
- +6590395715

Drug name: Epkinly(epcoritamab-bysp)
Drug alias:
English name: epcoritamab-bysp
R&D company: AbbVie Company
Indications: It is used to treat adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL).
Model specification: 4mg/0.8ml, 48mg/0.8ml.
Epkinly is the first and only bispecific antibody in which T cells participate, and it is made by Genmab’s proprietary double antibody technology. Genmab’s double antibody -CD3 technology aims to selectively guide cytotoxic T cells to trigger an immune response against target cell types. It aims to bind CD3 on T cells and CD20 on B cells at the same time, and induce CD20+ cell killing mediated by T cells.
Epkinly(epcoritamab-bysp)_ Hong Kong Jimin Pharmaceutical
[Epkinly indications]
Epkinly is used to treat adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL), and it is not specified otherwise (NOS), including DLBCL caused by inert lymphoma and advanced B-cell lymphoma after two or more systemic treatments. Epkinly is approved according to the FDA’s accelerated approval plan, which is based on the response rate and durability of the response. The continued approval of this indication may depend on the verification and description of clinical benefits in confirmatory trials.
[Epkinly recommended dosage and administration method]
First, important administration information
1. Use Epkinly for patients with sufficient water.
2, in the first cycle before each drug administration.
3. Epkinly should only be used by doctors with medical support to control serious reactions, such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
4. Inject Epkinly subcutaneously according to the dosage in Table 1 to reduce the incidence and severity of CRS. Because of the risk of CRS and ICANS, patients should be hospitalized for 24 hours after taking 48 mg on the 15th day of the first cycle.
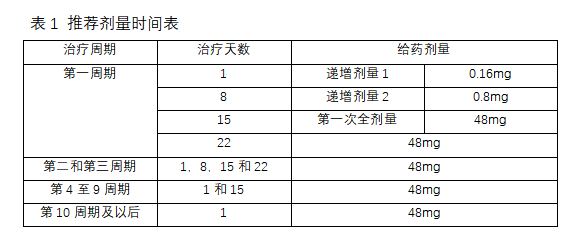
Second, the recommended dose
Epkinly is only used for subcutaneous injection. Table 1 provides the recommended dosage plan of Epkinly. Epkinly is administered in a cycle of 28 days until the disease progresses or the toxicity is unacceptable.
Epkinly(epcoritamab-bysp)_ Hong Kong Jimin Pharmaceutical
[Epkinly used in special people]
1. Pregnancy
According to the mechanism of action, pregnant women taking Epkinly may cause harm to the fetus. There are no available data on pregnant women using Epkinly to assess drug-related risks. Epcoritamab-bysp has not been used to study animal reproductive or developmental toxicity. Epcoritamab-bysp caused T cell activation and cytokine release; Immune activation may impair pregnancy maintenance. In addition, based on the expression of CD20 on B cells and the discovery of B cell depletion in non-pregnant animals, EPCORITAMIB-BySP can lead to the decrease of B cell lymphocytes in infants exposed to EPCORITAMIB-BySP in utero. It is known that human immunoglobulin G (IgG) crosses the placenta; Therefore, it is possible for Epkinly to be transmitted from mother to developing fetus. Inform women of the potential risks to the fetus.
2, lactation
There is no information about the existence of epcoritamab-bysp in breast milk, its effect on breast-fed children or milk yield. Because maternal IgG exists in breast milk, and the absorption of epcoritamab-bysp may lead to serious adverse reactions in breast-fed children, it is suggested that women should not breastfeed during Epkinly treatment and within 4 months after the last administration.
3. Patients with reproductive potential
Pregnant women taking Epkinly may cause harm to the fetus. Verify the pregnancy status of women with reproductive potential before starting Epkinly. Women suggest that women with reproductive ability use effective contraceptive methods during Epkinly treatment and within 4 months after the last administration.
4. Pediatric medication
The safety and efficacy of pediatric patients have not been determined.
5. Medication for the elderly
In clinical trials, 49% of patients with recurrent or refractory LBCL treated by Epkinly were aged 65 or above, and 19% were aged 75 or above. Compared with young patients, there is no clinical difference in the safety or efficacy of patients aged 65 or above.
[Epkinly Adverse Reaction]
The most common side effects of Epkinly include CRS, fatigue, muscle and bone pain, injection site reaction, fever, stomach (abdomen) pain, nausea and diarrhea.

CareMed Pharmaceutical Limited is a leading provider of trading services for the importation, marketing, and distribution of healthcare products nationwide.
© 2024 CareMed Pharmaceutical Limited · Developed By Channel Soft Solution