[ELREXFIO indications]
ELREXFIO(elranatamab-bcmm) is used to treat adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least four treatment regimens, including proteasome inhibitors, immunomodulators and anti-CD38 monoclonal antibodies.
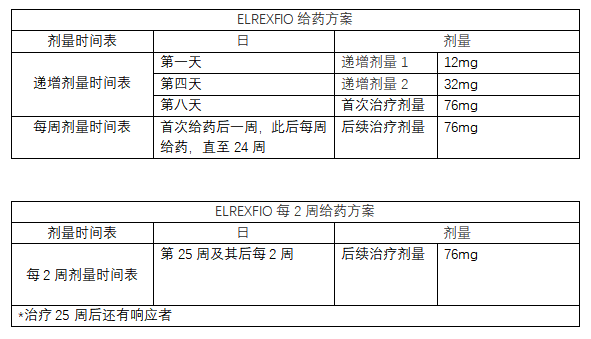
[recommended dose of ELREXFIO]
ELREXFIO is only used for subcutaneous injection. ① Take pre-treatment drugs as recommended. ② Patients should be hospitalized for 48 hours after the first incremental dose and 24 hours after the second incremental dose.
[ELREXFIO Warnings and Precautions]
1. Infection: It can lead to serious, life-threatening or fatal infection. Monitor the signs and symptoms of infection in patients and carry out appropriate treatment. Patients with active infection are not treated. 2. Neutropenia: monitoring the whole blood cell count at baseline. 3. hepatotoxicity: it can lead to the increase of ALT, AST and bilirubin. According to clinical indications, liver enzymes and bilirubin were monitored at baseline and during treatment. 4. Embryo-fetal toxicity: it may cause harm to the fetus. It is suggested that women take effective contraceptive measures.
[ELREXFIO is used in a specific population]
Breastfeeding: It is not recommended to breastfeed.
[Adverse reaction of ELREXFIO]
The most common adverse reactions (incidence ≥20%) are CRS, fatigue, injection site reaction, diarrhea, upper respiratory tract infection, musculoskeletal pain,
Pneumonia, loss of appetite, rash, cough, nausea and fever. The most common 3-4 laboratory abnormalities (≥30%) are lymphopenia, neutropenia, hemoglobin reduction, leukopenia and thrombocytopenia.